
Small project
The inhibitor of matriptase and prostasin (a similar serine protease) HAI-2 was investigated by MSc student Klaudia Parsberg from april to july 2022.
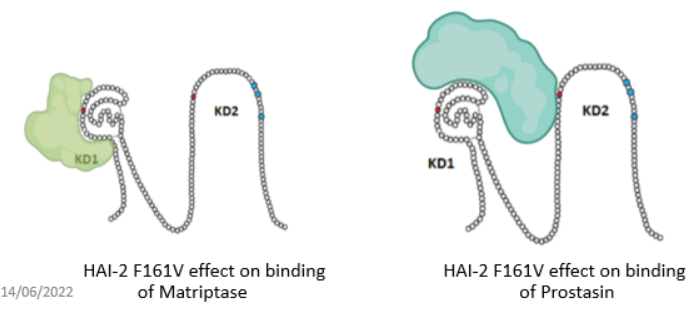
The purpose was to investigate the binding of matriptase and prostasin to wildtype HAI-2 and HAI-2 with two mutations in kunitz-domain 2 og HAI-2 essential for the folding and inhibitory effect on prostasin but not matriptase-
The study was conducted due to the interplay between these three proteins, where matriptase and prostasin activate each other by catalytic cleavage where wildtype HAI-2 inhibits both.

Conclusion
The conclusion from these experiments is that prostasin and matriptase show competitive binding to wildtype HAI-2, where prostasin outcompetes the binding of matriptase to some extend.
On the other hand both mutations (F161V and Y163C) in kunitz domain 2 of HAI-2 leads to a loss of the ability of prostasin to outcompete matriptase from bining to mutated HAI-2.
This indicates that matriptase and prostasin do not bind simultaneously to the some HAI-2, but binds seperately to different molecules og HAI-2.
