Matriptase
Matriptase is expressed in all types of epithelial cells, and dysregulation of matriptase is implicated in both epithelial-derived cancer and breast cancer invasion and metastasis.
The role and cellular mechanisms of matriptase is poorly understood, allong with the knowledge of it´s regulators, the HAI proteins.
With that being said, evidence shows that matriptase proteolytically activates at least two procarcinogenic
signal transduction pathways. Firstly, matriptase affects c-Met pathway activation, and the phosphorylation of c-Met, Gab1 and Akt. Secondly, it stimulates the receptor PAR2 which is involved in inflammation and pain, indicating that PAR2 is a substrate for matriptase.
Matriptase is an enzyme of the type called serine proteases, or serine peptidase.
Matriptase cleaves various proteins and peptides and in particular small side chain amino acids, such as the two oppositely charged amino acids Arg, Lys, as well as the non-polar aliphatic amino acids Ala and Gly.
Matriptase activates e.g. the hepatocyte growth factor, and urokinase plasminogen activator. Therefore, the function of Matriptase suggested to by activation of other proteases and latent growth factors of the epithelium.

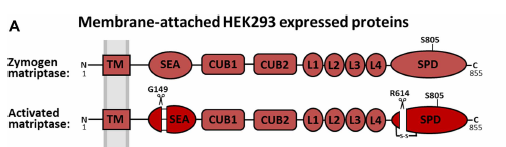
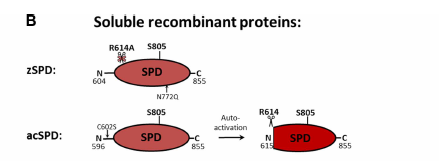
Structural representation of matriptase
A structural representation of the active zymogen form (non-SEA cleaved) and the activated (SEA cleaved) form of matriptase is shown in figure A, with the soluble recombinant forms of the catalytic SPD (serine protease domain) shown in figure B.
